Whistleblowing is good for our health. Not necessarily the whistleblower’s health. But, while we’ve been watching the battle over whistleblower protection in Washington, insiders have been busy flagging health fraud.
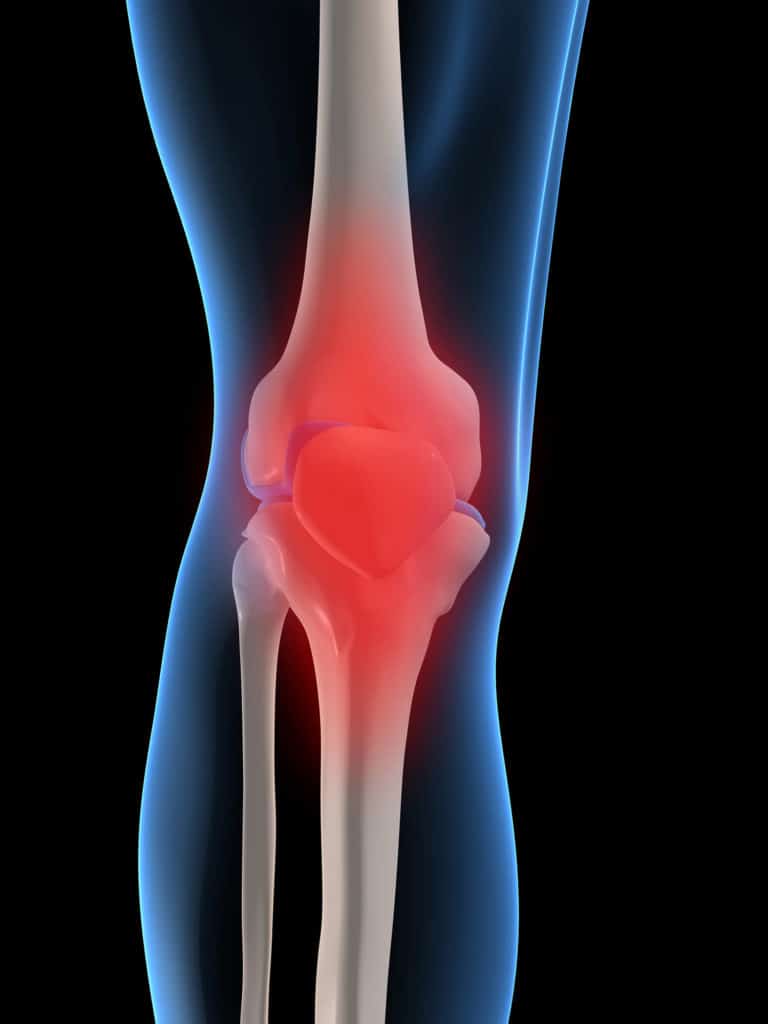
In its October announcement of a $7.1 million settlement with the now defunct Osteo Relief Institutes, the Department of Justice (DOJ) describes viscosupplementation as a treatment for osteoarthritis “in which a doctor injects a gel-like fluid into a patient’s knee joint to act as a lubricant and to supplement the natural properties of joint fluid.”
But the clinic didn’t quite get it right, according to the DOJ.
The government alleged that these clinics administered viscosupplementation injections to patients who did not need them, used multiple brands of viscosupplements successively on patients without clinical support, and used discounted viscosupplements reimported from foreign countries. The government also alleged that they provided unnecessary custom knee braces to patients.
The company had clinics nationwide, including one on the Jersey Shore. The Asbury Park Press has been following the case, which has led to lawsuits from patients who say they developed infections.
In Iowa, surgeon Wilson Asfora is facing his second round of charges of profiting from unnecessary care In late October, Sanford Medical Center agreed to pay $20.25 million to settle allegations of kickback and unnecessary surgery. From the DOJ:
The settlement announced today resolves allegations that Sanford knew that one of its top neurosurgeons was improperly receiving kickbacks from his use of implantable devices distributed by his physician-owned distributorship (POD). Sanford allegedly received warnings from the neurosurgeon’s physician colleagues and others about the alleged kickback scheme and was aware of the heightened compliance risks associated with POD
The settlement stems from a lawsuit filed by two whistleblowers, who will receive $3.4 million of the settlement. According to the announcement of settlement, they tried internal reporting and didn’t get anywhere.
Sanford allegedly received warnings from the neurosurgeon’s physician colleagues and others about the alleged kickback scheme and was aware of the heightened compliance risks associated with PODs. In addition, the neurosurgeon’s colleagues and others repeatedly warned Sanford that the neurosurgeon was performing medically unnecessary procedures involving the devices in which he had a substantial financial interest. The United States alleged that, despite these repeated warnings, Sanford continued to employ the neurosurgeon, continued to allow him to profit from the devices he used in surgeries performed at Sanford, and continued to submit claims to federal healthcare programs for these surgeries, including procedures that were medically unnecessary.
The Argus Reader has been reporting on the case. At one point, they obtained an email from hospital management to employees that shamed the whistleblowers.
“The complaint in question is based on second guessing Dr. Asfora’s professional relationship with Sanford and the care he provided to Sanford patients, years after the fact,” the email says. “It’s a shame that these ‘whistleblowers’ and the government have chosen to interject themselves into the relationship between these patients and their doctor and hospital… No salacious news article will ever accurately capture the lengths our clinicians go to, every day, delivering the best possible patient care. Sanford will have its day in court and will vigorously defend against the allegations asserted in the complaint.”
Here’s what hospital management told the paper after the settlement. They are still defending the quality of his care, but they’ve agreed to work with the feds on the case against “co-defendants.”
“As you know, Sanford took these allegations seriously, including suspending the use and purchase of Dr. Asfora’s devices,” Hocks wrote. “We then severed our relationship with him, based solely on his business practices – not his medical care. We are confident that Sanford acted appropriately and in good faith in the conduct of this matter, and we believe collaborating with the federal government on this settlement was in the best interest of our patients.”